

Orders by our customers, even when based on our offers, shall become binding only when confirmed in writing by us or when delivered.ĭue to rapid delivery basically no cancellation of orders is possible. Our offers and list prices are subject to alteration. We do not recognize the general conditions of our customers, even if we do not explicitly reject them in a specific case. If we act as agents of other companies, particularly of Merck KgaA, Darmstadt, the General Conditions of this company shall apply to the procured business (the so-called extended retention of title with regard to claims resulting from the sale of goods which remain the property of VWR until fully paid is, in view of the Austrian laws, considered as not agreed upon for deliveries to Austria) If a business has been concluded on the basis of the present General Terms and Conditions of Sale and Delivery, these will apply also to further business, even if no reference is made to them. By placing an order the customer irrevocably accepts the present conditions whose modification requires our written consent. particularly to industrial chemicals, reagents and diagnostic products as well as general laboratory supplies including equipment for the use of these products and consulting.

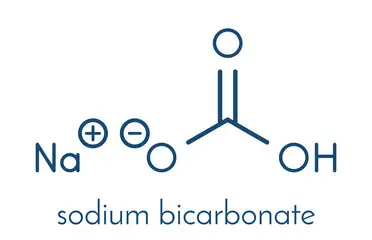
Sodium hydrogen carbonate formula skin#
However, eye or skin contact with the concentrated NaHCO 3 solutions can cause severe eye and skin irritation.The present general terms of sale and delivery apply to all business of VWR International GmbH, i.e. Health effects/safety hazards: It is not considered toxic or hazardous. It is also used as a fire retardant, in fireworks, for mild disinfecting, pest control, pH balancing, paint removal and metal cleaning applications. Uses: Sodium hydrogen carbonate is commonly used as an antacid, in baking powder, as odor absorber, drying agent, and in toothpastes. It also reacts with bases such as sodium hydroxide to form sodium carbonate: It decomposes upon heating into sodium carbonate, water and carbon dioxide: NaHCO 3 + HCl → NaCl + H 2CO 3 → H 2O + CO 2 Sodium hydrogen carbonate reacts with acids to give salts and carbonic acid, which then decomposes into carbon dioxide gas and water: Its melting point is 50 ☌, and it is highly water soluble.Ĭhemical properties: NaHCO 3 dissolves in water to give weakly acidic carbonic acid (H 2CO 3) and the basic hydroxide ions, making it an overall weak base. It is also commonly available as a fine white powder, with a density of 1.2 g/mL. Physical properties: Sodium hydrogen carbonate is an odorless, white crystalline solid with a density of 2.2 g/mL. NaCl + NH 3 + CO 2 + H 2O → NaHCO 3 + NH 4ClĪnother main preparation method involves dissolving soda ash (sodium carbonate mineral) in water and passing carbon dioxide through the solution. Preparation: Sodium hydrogen carbonate is also prepared on large scales by the Solvay process, in which sodium chloride reacts with ammonia, carbon dioxide and water to give NaHCO 3 along with ammonium chloride salt (NH 4Cl). Occurrence: Sodium hydrogen carbonate occurs naturally as the mineral nahcolite, which is found in many mineral springs. It is an ionic compound made of sodium cations (Na +) and bicarbonate anions (HCO 3 -). Formula and structure: The chemical formula of sodium hydrogen carbonate is NaHCO 3, and its molar mass is 84.007 g/mol.


 0 kommentar(er)
0 kommentar(er)
